How Planck found the Quantum
Your October 2002 article in "This Month in Physics History" gives an excellent account of how Planck obtained his famous formula for black-body radiation by fitting experimental data to an expression based on entropy arguments. But like many other accounts on this subject, this article does not offer even a clue as to how Planck came upon the idea of introducing the concept of energy quanta to justify his empirical formula. Did Planck make this revolutionary discovery by just "fiddling around", as Feynman tells the story, or by "divine" madness, according to Abraham Pais?
Actually, the answer can be found from a reference in his December 14, 1900 paper where he first announced his quantum hypothesis. There Planck cited Boltzmann's 1877 paper on the relation between entropy and probability. To illustrate this relation, which involves counting the number of configurations of a system, Boltzmann had introduced a one dimensional molecular gas with discrete energies defined by integer multiples of a fundamental element of magnitude 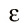
Of course, in the next section of his paper Boltzmann took the limit that 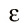
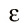
Michael Nauenberg
Santa Cruz, California
Planck Discovered Boltzmann's Constant
In the October "This Month in Physics History", Max Planck's formula for black body radiation was featured. Although the author says that Planck "made no other significant discoveries of comparable importance to his 1900 work," he presents only part of the significant discovery. Besides the constant h, Planck also discovered the constant k, known today as the "Boltzmann constant." The values he calculated were amazingly close to the present day values.
At the presentation of the Nobel Prize in Stockholm in June 1920, Planck commented that to his knowledge, Boltzmann never realized that k had a unique value; he never even thought of a possible measurement of such a constant. This discovery of k should rank as a major discovery in physics, although it was a by-product of the derivation of the radiation theory, published in the same paper.
Henn H. Soonpaa
Grand Forks, North Dakota
In Defense of Rare Earth Metals
I would like to respond to the article by Martin Bridge in the October issue of APS News. I agree with the author that we should verify the discovery of new elements. Likewise, we should verify that of new compounds and chemicals that claim to have special properties.
However, the author goes on to suggest that many of the rare earth metals are not new elements, but just "copycats" of other elements, or possibly some exotic carbon structures. This is a very misleading idea. If several of the rare earth metals are "copycats," then why does x-ray diffraction reveal that many of these elements exist in different structures at ambient conditions? Other properties of rare earth metals have also been examined in great detail to show their distinct differences.
Finally, the author claims that "Nobody will really miss them." REMs are used to enhance the properties of iron and other metals to increase their strength and operating life. They are used in glassmaking, ceramic glazes, glass-polishing abrasives, catalysts for petroleum refining, lasers, and color-television picture tube phosphors, among other applications. So maybe we would miss them.
Gary Chesnut Los Alamos,
New Mexico
Not Convinced that Silicon Doesn't Exist
I find it difficult to give Martin Bridge's article, "Investigation Pokes Holes in the Periodic Table," (APS News, October 2002) much credibility when he seems to quote for the most part those who do not wish to be identified. I would like to see the evidence that silicon does not exist. Failure to quote sources is hardly the scientific method. Clarence Cunningham Stillwater, Oklahoma
Ed. Note: Martin Bridge's article appeared under the banner "Zero Gravity: the Lighter Side of Science". It was not meant to imply that the existence of any chemical elements other than 118 and 116 had actually been called into question. APS News regrets any confusion that may inadvertently have arisen.
Fraud Not A Subject for Humor
With regard to the October 2002 Zero Gravity Column in APS News, it's hard for me to imagine something that stems from the falsification of data in relation to the claimed discovery of elements 118 and 116 as being related to "The Lighter Side of Science".
Related to Enron, Arthur Andersen and all the present day failures of personal and institutional ethics, yes. Relating to a tragedy for individuals and for the profession, certainly. But hardly a subject for humor.
David J. Ritchie
Naperville, Illinois
Give Credit Where Credit is Due
The response of the APS via its public affairs panel to the two recent scandals is a little like somebody asking CEOs after the recent corporate scandals to be "nice". Authorship guidelines in any science and in physics in particular are by design unenforceable. The authorship ethical guideline of the APS, compared to its biomedical sisters, is so ill defined that physicists themselves cannot agree on what it means, so ill marketed that 74% of junior physicists claim not to have seen them and 92% of APS members claim not to use them.
Inappropriate coauthorship is common among physicists. Public research monies have an extra tax added that moves money from those physicists who did the research to those physicists who pretend they did. If the APS wants to clamp down on inappropriate coauthorship, we can. We can decide to give back to the individual the incentive to do work, to reward creativity and not politics. We can decided to give credit where credit is due. But I guess we won't.
Eugen Tarnow
Riverdale, New York
©1995 - 2024, AMERICAN PHYSICAL SOCIETY
APS encourages the redistribution of the materials included in this newspaper provided that attribution to the source is noted and the materials are not truncated or changed.
Associate Editor: Jennifer Ouellette
December 2002 (Volume 11, Number 12)
Articles in this Issue

