Cecilia Payne-Gaposchkin
Have you ever wondered why stars shine? When you gaze up at the night sky and observe the twinkling shapes, or bask in the warmth of the sun on a hot afternoon, have you pondered the source of that light and heat? To start figuring it out, your first instinct might be to explore what a star is made of. However, doing so is hard, because stars are really *really* far away. Even our nearest star, the sun, is still really far away. Also, in the event you were able to travel through space and get close enough to touch a star, your spaceship would melt from the extreme heat (note: this is not an ideal situation for doing science).
Given these challenges, for a long time the main theory among astronomers was that all stars are made of the same stuff as the Earth: mostly silicon and oxygen, the primary components of rocks. To get the real answer though, it took someone looking at the question of stellar composition in a new light.
In the early 1920’s, Cecilia Payne was a student attending Newnham College at the University of Cambridge in the United Kingdom. She initially studied botany, though was interested in many scientific fields and also took coursework in physics and chemistry. She was motivated to begin studying astronomy after attending a lecture by Sir Arthur Eddington. Eddington had led an expedition to observe the solar eclipse of 1919, providing one of the first confirmations of Einstein’s general theory of relativity by measuring the bending of starlight by the sun’s gravity. To Cecilia, hearing Eddington talk about his research was a transformative experience. She decided she was done with botany, and to be “dedicated to physical science, forever.”
Cecilia dove into the study of physics, making great use of the many resources for her to learn at Cambridge University. She attended lectures on atomic structure by Niels Bohr, conducted experiments in courses taught by Ernest Rutherford, and spent many late nights working at the Newnham observatory. which she described as “a pure delight”, though there were several aspects which made the work more difficult for her than for others. In some classes Cecilia was the only woman, and she often faced harassment in lectures and derision in the lab. She remarked feeling like a “second-class student”, and at times had trouble finding good mentors. Despite these challenges, Cecilia persevered, and her strong ambition was recognized by her professors.
After Cecilia graduated from Newnham, she knew she wanted to continue working in astronomy. However, there were few opportunities for women to pursue a career in science in the United Kingdom at the time. One of her peers suggested she go to America, where there was an open position to study at the Harvard observatory in Massachusetts. With several letters of recommendation written by the faculty of Cambridge University, she was accepted into the Harvard program and traveled to America in 1923.
When Cecilia arrived at the observatory, the director suggested she take on a similar role as the other women working there. The Harvard observatory had a history of employing women as ‘computers’, or people who take measurements and do calculation work. Not being able to physically touch the stars, they were instead analyzing starlight. Over several decades of hard work from the computers, the observatory had amassed the largest collection of stellar observations in the world, with millions of individual measurements. However, Cecilia had a different plan in mind, and since she had acquired her own source of funding through a fellowship she was able to construct a research plan all her own.
Cecilia’s background in physics and chemistry gave her a deep understanding of what information can be obtained by collecting the light from stars. She knew that in the same way that you can differentiate objects by what color they are (grass is green, snow is white, oranges are orange), the light coming off an object can tell you a great deal about it. In particular, Cecilia was interested in looking at a star’s emission spectra.
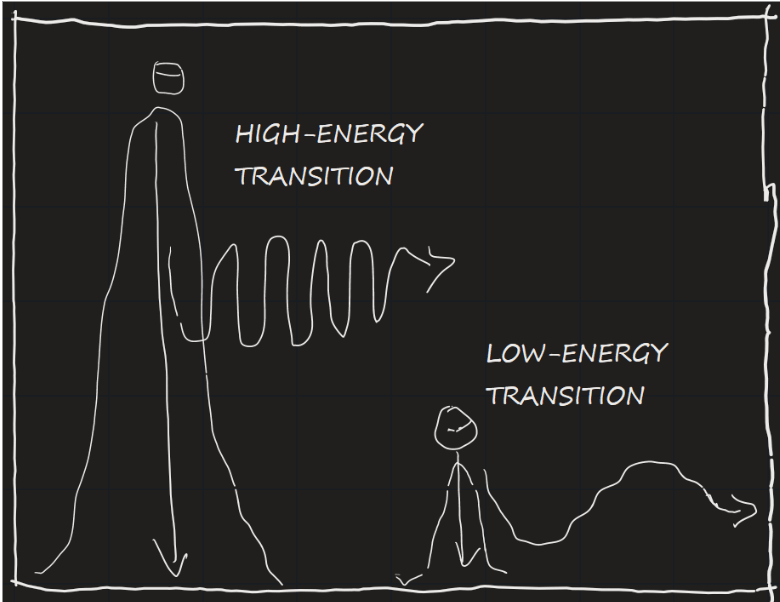
Everything around us is made of tiny particles called atoms, and each atom consists of a dense, positively charged center called a nucleus, surrounded by lighter, negatively charged particles called electrons. Opposite charges attract so electrons are pulled towards the nucleus, which means they need energy to get further away. However, because of some *really cool* stuff called quantum mechanics, only certain positions, or energy levels, are allowed for the electrons to occupy. This means that an electron can only make distinct - or quantized - jumps between energy levels. If an electron goes from a higher energy level to a lower one, it emits the energy difference as a packet of light called a photon. The energy of a photon is related to its frequency, and we can tell different frequencies of light apart because they have different colors. Further, different types of atoms, known as elements, have varying amounts of positive charge in their nuclei which dictate the allowed energy levels of electrons. So, each element has a unique set of colors of light, or emission spectrum, associated with it.
At the observatory, the researchers measured the spectra of light emitted by elements in a star using a device called an ‘objective prism’. This prism separates the incoming light of the star into all its component colors, and if there is a lot of a particular element its emission spectrum shows up as a set of very intense lines. These lines were then captured on large photographic plates for later analysis. Cecilia knew that by comparing the intensity of light at each energy level in the emission spectrum, one should be able to determine the relative abundance of elements in a star. This was known by other astronomers of the time, too, but why weren’t they getting the right answer?
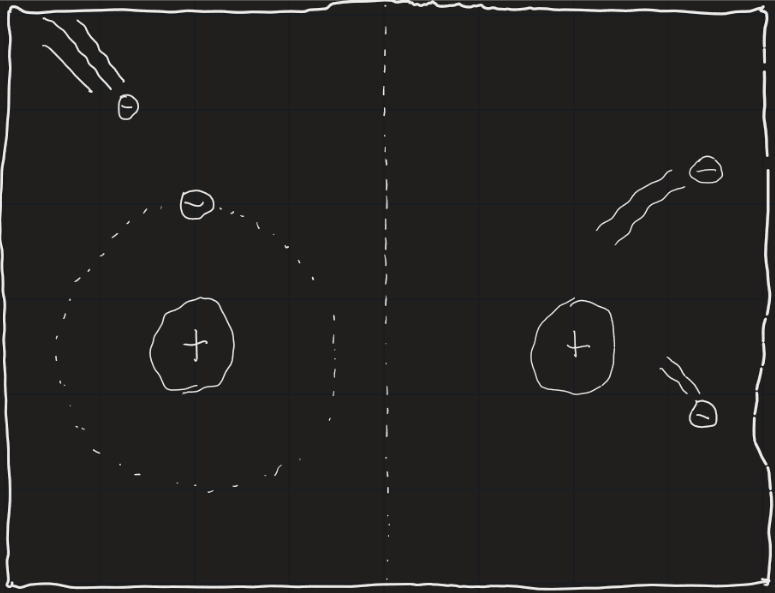
Well, an electron in an atom can only drop down an energy level and emit a photon if it’s bound to the atom in the first place. This means the intensity of the spectral lines can change due to ionization (when an atom loses an electron). If too many atoms of a particular element are ionized and have no electrons, the corresponding spectral lines will be fainter.
Ionization can occur whenever an electron gains enough energy to completely leave the attraction of the nucleus. This energy could come in many forms, one of which is heat. Temperature is essentially a measurement of how fast particles in a material are moving, which means if something is hot its particles are moving fast. If something is very hot, some of its electrons can be moving so fast that when they collide with another electron that’s bound to a nucleus, that electron could get kicked away. On Earth, ionization is negligible. But on stars, a significant amount of atoms could be ionized (stars are HOT). The question was, how much of a difference does it make?
Cecilia thought a theory developed by Indian physicist Meghnad Saha in 1920 could hold the answer, and decided to test it. Saha had formulated an equation that related the physical conditions of stars to the observed spectral lines from the elements. His theory, known as the Saha ionization equation, showed how the temperature and density of a star’s atmosphere could determine the ionization states of various elements. Using the Saha equation, one could correct for the effects of ionization and deduce the actual relative abundance of elements in stars.
Cecilia was in a very special position: she understood the physics, knew the theories, and had access to the data. All it took was the insight to put the pieces together.
What she found was astounding. For most of the elements, Cecilia concluded they were in quantities that roughly agreed with the prevailing theory. There were two important outliers though: Helium was several thousand times more abundant than expected, while Hydrogen was over a *million* times more abundant than expected.
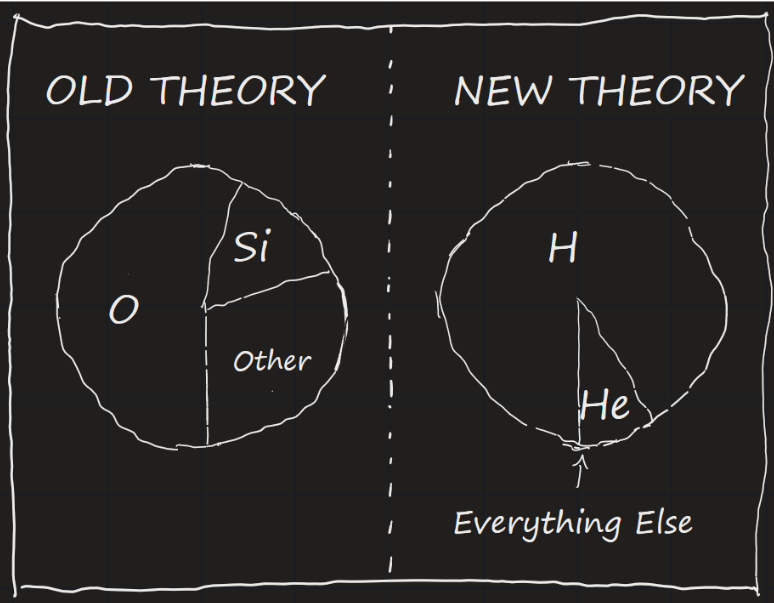
This put Cecilia in a tight spot. Her discovery was groundbreaking, but it went against the theories of the most prominent astronomers of the time. She needed their approval to publish her thesis and earn her doctorate. As such, Cecilia was forced to describe her own findings as “spurious” and “almost certainly not real”.
Almost.
After her thesis was published in 1925, Cecilia continued to work and study at the Harvard observatory. It may not have been immediately accepted for the brilliance it was, but after her results were reproduced by independent observation her work did gain some recognition in the astrophysics community. Despite her contributions and dedication, her standings at Harvard were not much improved. She was chronically underpaid, not given equal credit for equal work, and was often passed up for influential positions in the observatory. She was only granted the title of professor in 1956, after lecturing for over 20 years! While these challenges were difficult to overcome, Dr. Cecilia Payne-Gaposchkin was nonetheless able to build a very successful career as an astronomer, a scientist, and a scholar. In her words, “It has been a case of survival, not of the fittest, but of the most doggedly persistent.”
Dr. Cecilia Payne-Gaposchkin’s thesis is now widely recognized as one of the most significant discoveries in the history of astrophysics. By demonstrating that stars are mostly hydrogen, she helped prove that stars are powered by nuclear fusion. At the immense pressure in the core of a star, hydrogen atoms are fused together to make helium (and eventually heavier elements), releasing an enormous amount of energy in the process.
So the next time you gaze up at the night sky, think of the pioneer whose work unveiled the secrets of the stars, expanding our understanding of the cosmos.